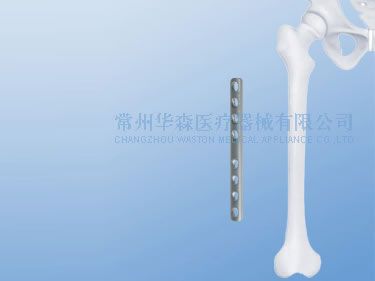
Femoral DCP, Dynamic Compression Plate
Parameters
| Product Name | Ti Product No. | SS Product No. | Thickness(mm) | Holes | Length(mm) |
| DCP for Femoral Limbs | 10203-007 | 20203-007 | 5 | 7 | 126 |
| 10203-008 | 20203-008 | 5 | 8 | 142 |
| 10203-010 | 20203-010 | 5 | 10 | 174 |
| 10203-012 | 20203-012 | 5 | 12 | 206 |
| 10203-014 | 20203-014 | 5 | 14 | 238 |
| Note: Used for femoral shaft fracture with HA4.5 cortical screws and HB6.5 cancellous screws. |
Materials: Pure Titanium, stainless steel. We can choose the raw materials according to the client's requirement.
Package: One piece per package
Package and Sterilization Treatment
The product is supplied in the non-sterile package. After originally packed with the paper plastic package, the product will be put into the plastic bag together with the Operation Instructions and the Product Qualification Certificates.
The paper plastic roll bag is used as the inner package. It is compounded from the medical dialysis paper and plastic film. The production environment is controlled with the cleanness of Class 10,000. In the cleaning shop, the package bag is cut according to the product size. Then, its one side will be sealed with the heat-sealing machine. After the bag has been used to contain the product, its other side needs to be sealed with the sealing machine.
The paper plastic, into which the product has been put, will be cleaned in the controlling area where the cleanliness is of Class 10,000. All the cleaning methods have been inspected. The product packed with this method needs no cleaning prior to use. It just needs to be treated with sterilization in accordance with the methods specified in the Product Instructions.
Related Names
Orthopedic Surgical Implants | Titanium Surgery Plate | Fracture Plate
Related products
Other products