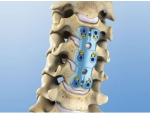
Spine
Our spine product offers an effective solution to the spine related problems, including the fracture, deformation of spinal column, intervertebral disc disease, and other factors caused back pain. This product chiefly includes anterior cervical plate system, NEULEN cervical laminoplasty plate system, M6 posterior cervical spinal screw-rod system, M7 series intelligent spinal screw-rod system, and so on.
Ⅰ. Contraindications
1. Abnormal bony structure
2. Anatomic abnormality of the nerve canal
3. Patients who are suffering from serious metal disorder or severe osteoporosis.
4. Patients who are allergic to metal.
Ⅱ. Precautions
1. The internal fixation instrument for the spinal column should be disposable.
2. If the implant has any bending or scratches during the surgery, its service life will be affected. The delayed union or nonunion may result in the loosening of the implant.
3. Whichever internal fixation instrument is difficult to bear the normal body burden alone, especially before the fracture healing. That is why the external fixation is necessary within 6 to 8 months after the surgery.
4. Weight bearing or the activities without the doctor's guidance, will lead to the loosening, bending, or fracture of the implant.
5. The time to remove the implant varies according to the patient's recovery after the surgery. Generally, the implant can be removed after 12 months or so.
6. After the surgery, the patient should periodically have the X-ray examinations according to the doctor's advice.
7. Only the orthopedic clinicians who have the attending physician qualifications are permitted to conduct the surgical operation.
Ⅲ. Usage
1. Prior to the surgery, decide the surgical plan on the basis of the imageological examination as well as the patient's symptoms and physical signs. Choose the proper implant and make sure the completeness of the surgical instruments.
2. Before the surgery, the implants and relevant surgical instruments must be treated with the high-temperature, high-pressure sterilization.
3. The operation should be carried out on the premise of the general anesthesia and the use of trachea cannula. Choose the appropriate position and incision. Reveal the spinous process, facet joint, and transverse process that need fixation. Then, the drilling and tapping can be implemented on the pedicle of vertebral arch. Select the reduction screw or U type screw of the proper size. For the whole surgical process, please refer to the Operative Orthopaedics.
Ⅳ. Potential Side Effect
1. The spinal dural breakage and the surgery caused nerve injury
2. The discomfort or pain resulted from the implant placement
3. Infection
4. Metal allergy or rejection reaction
5. The loosening or breakage of the implant caused by the delayed union or nonunion
6. Osteoporosis
Ⅴ. Maintenance and Storage
If the implant has any scratches or collision during the transportation and utilization, its strength and anti-fatigue performance will be thereby weakened. Therefore, you should store and use the implant in a proper way. Otherwise, the possible risks exist. The implant ought to be stored in the well-ventilated indoor environment with the relative humidity of no more than 80%. And the environment should be free from corrosive gas.
Ⅵ. Service Life
Before the fracture healing, the internal fixation acts as the main role to bear the physiological load. In general, the implant should be removed after approximately one year's healing. If not, the potential risk of secondary fracture and implant breakage will exist. Please see the doctor immediately, once you have any discomfort. After the fracture healing, that the implant breakage caused by the delayed removal, is regarded as the normal phenomenon.
Related Names
Spinal Implants | Internal Fixator | Interbody Fusion