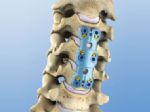
Anterior Cervical Plate System
The ACI anterior cervical plate system is a semi-constrained or semi-rigid system designed to offer the surgeon the possibility to intra-operatively choose the screw trajectory that best fits the individual patient anatomy presented. The 'load sharing' design concept accommodates the safe transfer of axial load to the bone graft without putting strain on either the screws or the plate as can occur in more constrained or rigid systems.
Plate thickness is an important consideration in protecting the patient against the risk of dysphagia. With a profile of 2.1mm, ACI is one of the lowest profile anterior cervical plates currently available today.
1. Variable Angle Semi-rigid Fixation System
The design elements, such as the ACI Cam-lock mechanism, ensure that all implants screws hold the plate securely in place and are prevented from backing out. The cams do not interfere with screw placement and do not add to the profile of the plate.
2. Numerous Screw and Plate Types for Surgeon Convenience
The ACI anterior cervical plate ystem includes 25 types plates-one level, two level and three level plates. Self-drilling and Self-tapping screws both are included in multiple lengths. Every implant option is at the surgeon's fingertips during the procedure.
3. Compact and User-friendly Instrumentation
In optimized designed instrumentation of ACI, every element is not only designed with ergonomics ideology, but also designed to work in concert with the whole system. For example, color coding of he drill match the screws color. All the instruments make the procedures more simple and conveniences.
4. Optimized by Size
The ACI anterior cervical plate has 2.1mm profile and 16mm width. The plate has contoured shape edges. The pre-curved design makes the cervical reconstruction as the normal curve.
5. Anti Skid Cleats
This product is used to resist plate movement during the holes preparation and screws insertion. The Character adds zero profile to overall construct-cleats "dig in".
Indications
1. Instability caused by trauma
2. Instability associated with correction of cervical lordosis and kyphosis deformity
3. Instability associated with pseudoarthosis as a result of previously failed cervical spine surgery
4. Instability associated with major reconstructive surgery for primary tumors or metastatic malignant tumors of the cervical spine.
5. Instability associated with single or multiple level corpectomy in advanced degenerative disc disease, spinal canal stenosis and cervical myelopathy.
Product Information
11101 Anterior Cervical Plate AC I
| Product No. | Holes | Length |
| 11101-(022~037) | 4 | 22/25/27/29/31/33/35/37 |
| 11101-(039~053) | 6 | 39/41/43/45/47/49/51/53 |
| 11101-(054~078) | 8 | 54/57/60/63/66/69/72/75/78 |
11102 Anterior cervical Screw
| Product No. | Diameter(mm) | Length |
| 11102-(112~120) | 4.5 | 12/14/16 /18/20 |
11102 Revision Screws
| Product No. | Diameter(mm) | Length |
| 11102-(312~318) | 4.5 | 12/14/16 /18 |
11102 Revision screws
| Product No. | Diameter(mm) | Length |
| 11102-(412~416) | 4.8 | 12/14/16 |
Package and Sterilization Treatment
The product is supplied in the non-sterile package. After originally packed with the paper plastic package, the product will be put into the plastic bag together with the Operation Instructions and the Product Qualification Certificates.
The paper plastic roll bag is used as the inner package. It is compounded from the medical dialysis paper and plastic film. The production environment is controlled with the cleanness of Class 10,000. In the cleaning shop, the package bag is cut according to the product size. Then, its one side will be sealed with the heat-sealing machine. After the bag has been used to contain the product, its other side needs to be sealed with the sealing machine.
The paper plastic, into which the product has been put, will be cleaned in the controlling area where the cleanliness is of Class 10,000. All the cleaning methods have been inspected. The product packed with this method needs no cleaning prior to use. It just needs to be treated with sterilization in accordance with the methods specified in the Product Instructions.
Related Names
Spine Fixation Set | Spinal Surgery Product | Neck Fusion Surgery
Related products
Other products